J.M. Guenet
Institut Charles Sadron, CNRS-Unistra, 23 rue du Loess, 67034 STRASBOURG Cedex2
Neutrons have been extensively used in the small angle range (SANS) for investigating the large-scale structures of (supra)polymers. Another interesting aspect of neutrons lies in their use in the wide-angle, or diffraction range for studying the crystalline organization of co-crystals obtained from polymers or supramolecular polymers with appropriate solvents (see figure 1 left). The generic name for these systems is “molecular compound” from the terminology used in temperature-concentration phase diagrams. Other names are often found such as crystallo-solvates, inclusion compounds, intercalates, clathrates, molecular complexes, and the like. These systems are often observed in thermoreversible gels from polymer and in organogels from supramolecular polymer [1]. As these (supra)polymers and associated solvents commonly contain hydrogen atoms, while their deuterated counterparts are readily available or can be synthesized, one may gain access up to four different neutron diffraction patterns for a binary system by toying with the isotopic labelling of each species. This feature is a decisive advantage over X-ray diffraction [1-3]. Neutrons are therefore either complementary to X-rays [2] or even are the only approach for investigating these compounds [3]. Indeed, neutrons are of great help in two situations for finding out whether a system is a molecular compound or not: 1) when the sample cannot be oriented, which stands as a major drawback for assigning X-ray diffraction peaks, a problem often encountered with supramolecular polymer organogels, and 2) when systems are prepared with highly halogenated solvents that absorb almost entirely X-ray photons, keeping in mind that these systems must not be dried so as to avoid the alteration of the original crystal structure (figure 1 right). Differing diffraction patterns obtained by isotopic labelling, namely appearance or disappearance of peaks and/or change in relative intensities, provide an unmistakable signature of the occurrence of a molecular compound [3]. Typical examples are displayed in figure 1 for the diffraction patterns of tri-aryl tris-amine/bromobenzene and tri-aryl tris-amine/tetrachloroethane compounds [3].


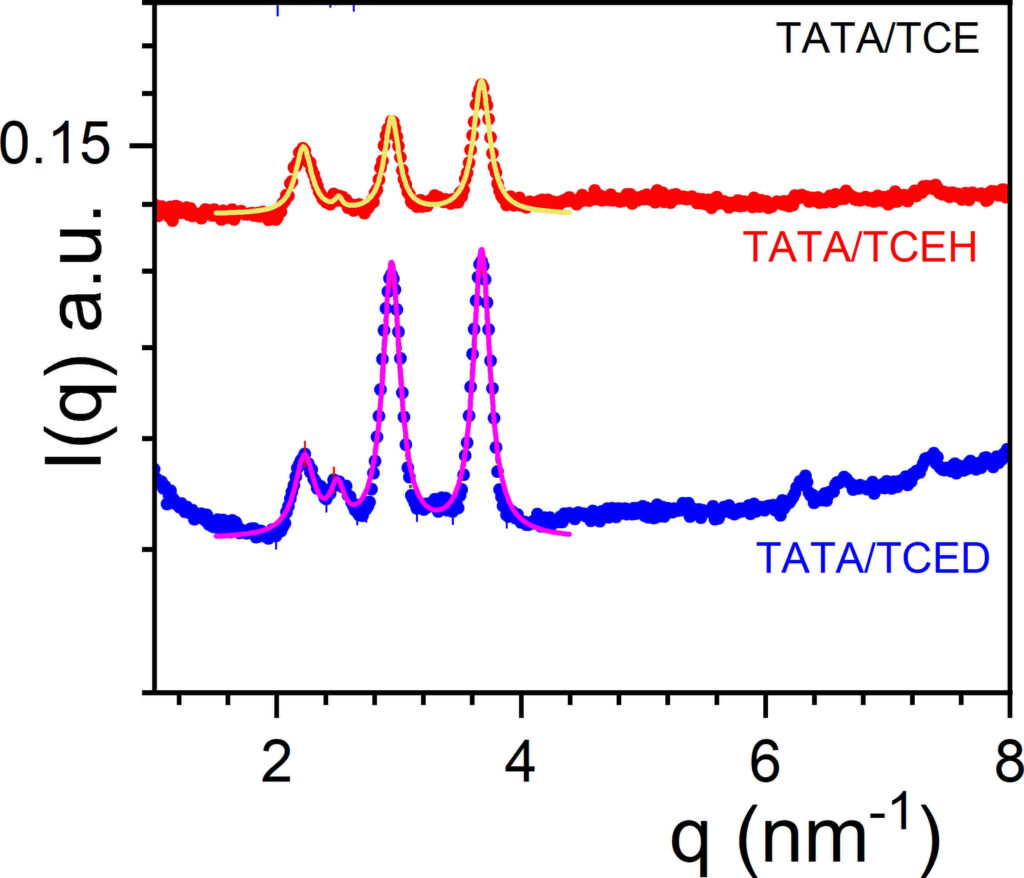
Figure 1: left: a typical molecular compound from poly(Ethylene-Oxide)/di-chlorobenzene as seen perpendicular to the polymer helical axis [1]; middle: diffraction patterns of tri-aryl tris-amine/bromobenzene systems; the use of deuterated bromobenzene allows one to reveal other diffraction peaks related to the solvent location within the compound; right: diffraction patterns of tri-aryl tris-amine/tetrachloroethane organogels, the intensities relative ratio differs whether a hydrogenous or a deuterated solvent is used [2], which allows one to conclude at least qualitatively of the occurrence of a compound. For this system, X-ray diffraction is not achievable due to the strong photon absorption [3]. Solid lines are fits with Lorentzian functions. Spectra obtained at ILL on D16 with the assistance of B. Démé.
[1] Guenet, J.M. Contribution of neutron diffraction to the study of crystallo-solvates (molecular compounds) from polymers and from supramolecular polymers Polymer 2024, 293, 126638, and references therein.
[2] Point, J.J.; Damman, P.; Guenet, J.M. Neutron diffraction study of poly(ethylene oxide) p-di-halogenobenzene crystalline complexes. Polym. Comm. 1991, 32, 477
[3] Guenet, J.M., Deme, B.; Gavat, O.; Moulin, E.; Giuseppone, N. Evidence by neutron diffraction of molecular compounds in tris-amide triarylamine organogels and in their hybrid thermoreversible gels with PVC. Soft Matter, 2022, 18, 2851











